This site is intended only for healthcare professionals resident in the Republic of Ireland
Menu
Close
Menu
Close

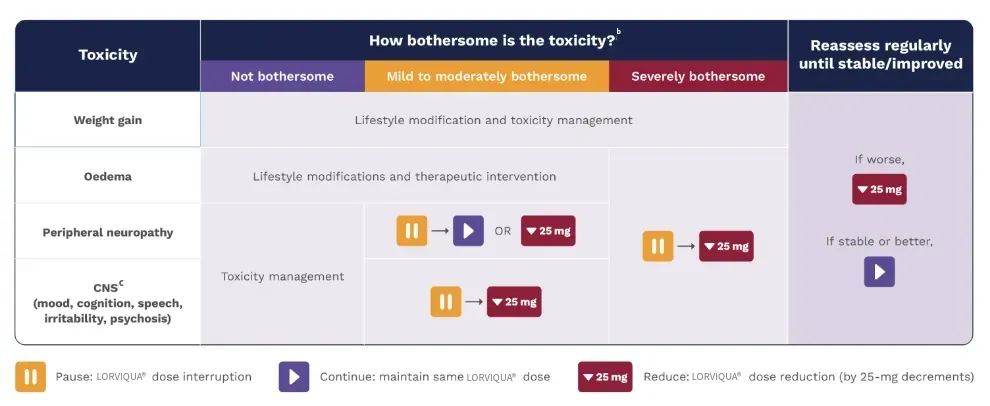
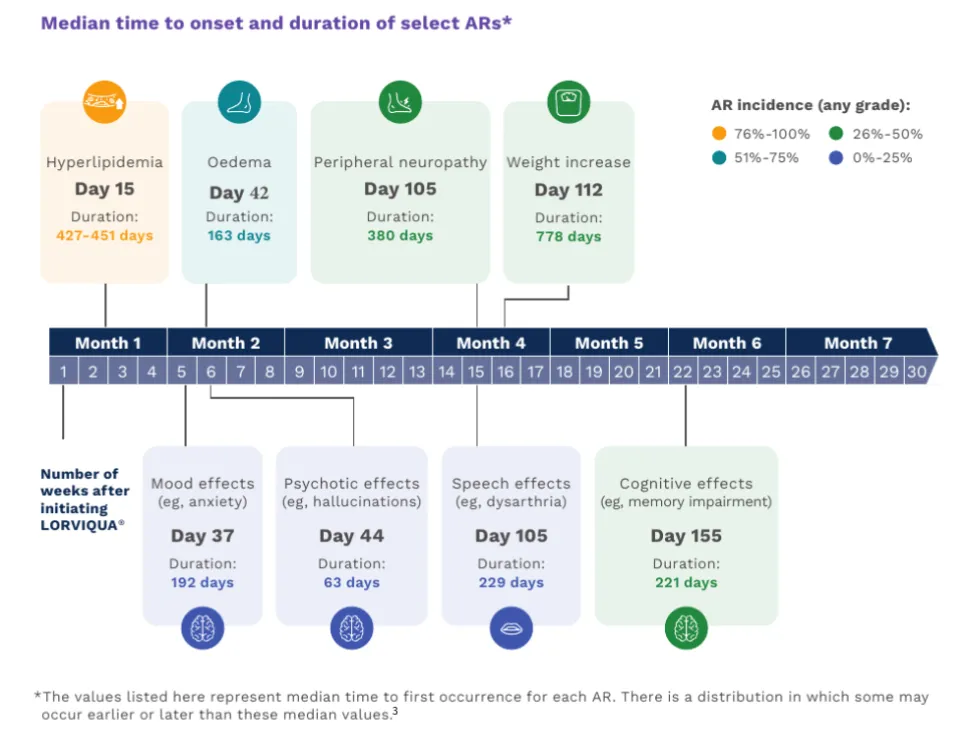
bAs severity increases, add management from left to right. For example, for edema that is severely bothersome, consider lifestyle modifications, therapeutic intervention, dose interruption, and dose reduction. Note that all adverse reactions are subjective; if a patient experiences a moderately bothersome adverse reaction that is functionally debilitating or functionally detrimental, this may be interpreted as being severely bothersome after discussion with patient and healthcare provider. This is particularly true for CNS toxicities, for which severely bothersome may equate to any CNS functional detriment.3
cCNS toxicities tend to be bothersome and less likely to respond to mitigation strategies; therefore, early dose reduction in combination with temporary dose interruption may be preferred, and dose escalation after the resolution of symptoms is not recommended.3
AR=adverse reaction; CNS=central nervous system; ULN=upper limit of normal.
1.Solomon BJ, Bauer TM, Mok TSK, et al. Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced,ALK-positive non-small-cell lung cancer: updated analysis of data from the phase 3, randomised, open-label CROWN study.Lancet Respir Med. 2023;11:354-366.
2.Solomon BJ, Liu G, Felip E, et al. Lorlatinib versus crizotinib in patients with advanced ALK-positive non-small cell lung cancer: 5-year outcomes from the phase III CROWN study.J Clin Oncol 42, 3400-3409 (2024)
3.Liu G, Mazieres J, Stratmann J, et al. A pragmatic guide for management of adverse events associated with lorlatinib.Lung Cancer. 2024;191:107535.
Adverse events should be reported.
If you wish to make a medical information inquiry or report an adverse event please contact Pfizer on 1800 633 363
or email Pfizer at [email protected] or visit www.PfizerMedicalInformation.ie
Report an adverse event to your national reporting system (HPRA Pharmacovigilance)
Please sign in or register to gain access to information relating to Pfizer medicines and vaccines, medical conditions, patient materials and services.
This site is intended only for healthcare professionals resident in the Republic of Ireland. If you are a member of the public wishing to access information on a specific medicine, please visit https://www.medicines.ie
This website is brought to you by Pfizer Healthcare Ireland Unlimited Company, The Watermarque Building, Ringsend Road, Dublin 4, Dublin, Ireland, D04 K7N3.Registered in the Republic of Ireland No. 127002. Directors: S. Miller (Managing) K. Stanton. Company Secretary: M. Byrne
Copyright © 2026 Pfizer Limited. All rights reserved.
The information on this site is reserved exclusively for healthcare professionals resident in the Republic of Ireland and contains promotional content.
I confirm that I am a healthcare professional* resident in the Republic of Ireland.
If you select 'No', you will be redirected to Pfizer.ie, where you will be able to access information on Pfizer Healthcare Ireland Unlimited Company.
*The IPHA Code definition of a healthcare professional is a person of any of the following classes: (i) Registered medical practitioners (ii) Registered dentists (iii) Registered pharmacists (iv) Registered nurses
Terms of use
PP-UNP-IRL-0901. July 2025
PP-UNP-IRL-0901. July 2025