This site is intended only for healthcare professionals resident in the Republic of Ireland
Menu
Close
Menu
Close
Time to IC progression: ITT population1
Data cutoff: 31 October 20231
- No new CNS progression events were observed between years 3 and 51
Time to IC progression: ITT population1
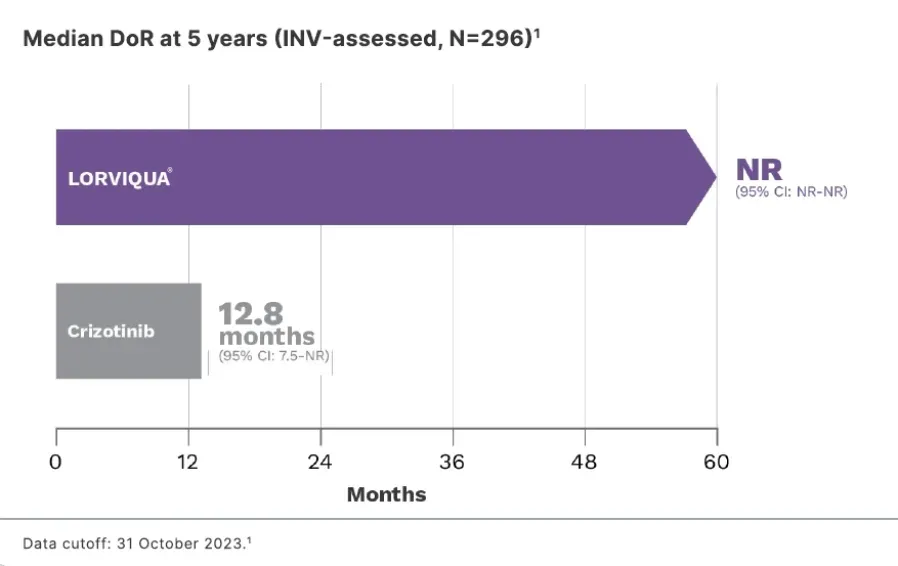
Data cutoff: 31 October 2023.1
- 96% of patients without baseline brain metastases were protected against CNS progression1
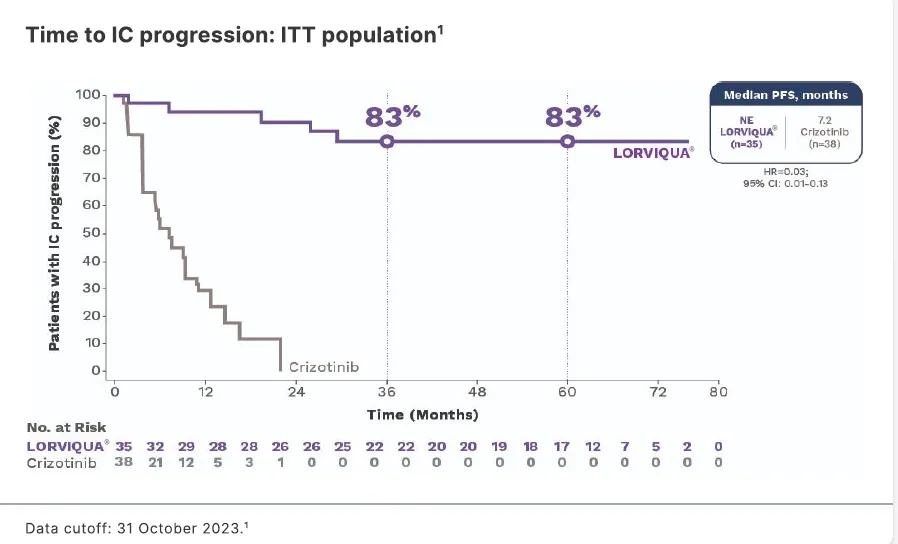
- 83% of patients with baseline brain metastases were protected against CNS progression1
†The primary endpoint of PFS was met in the CROWN trial primary analysis (median follow-up for PFS; 18.3 months for patients receiving LORVIQUA® and 14.8 months for patients receiving crizotinib); median PFS was not estimable for the LORVIQUA® arm. An unplanned follow-up analysis was performed at a median follow-up for PFS of approximately 60 months for patients on LORVIQUA® (55 months for patients on crizotinib) to confirm the effect of LORVIQUA® relative to crizotinib with longer follow up.
Adverse events should be reported.
If you wish to make a medical information inquiry or report an adverse event please contact Pfizer on 1800 633 363
or email Pfizer at [email protected] or visit www.PfizerMedicalInformation.ie
Report an adverse event to your national reporting system (HPRA Pharmacovigilance)
Please sign in or register to gain access to information relating to Pfizer medicines and vaccines, medical conditions, patient materials and services.
This site is intended only for healthcare professionals resident in the Republic of Ireland. If you are a member of the public wishing to access information on a specific medicine, please visit https://www.medicines.ie
This website is brought to you by Pfizer Healthcare Ireland Unlimited Company, The Watermarque Building, Ringsend Road, Dublin 4, Dublin, Ireland, D04 K7N3.Registered in the Republic of Ireland No. 127002. Directors: S. Miller (Managing) K. Stanton. Company Secretary: M. Byrne
Copyright © 2026 Pfizer Limited. All rights reserved.
The information on this site is reserved exclusively for healthcare professionals resident in the Republic of Ireland and contains promotional content.
I confirm that I am a healthcare professional* resident in the Republic of Ireland.
If you select 'No', you will be redirected to Pfizer.ie, where you will be able to access information on Pfizer Healthcare Ireland Unlimited Company.
*The IPHA Code definition of a healthcare professional is a person of any of the following classes: (i) Registered medical practitioners (ii) Registered dentists (iii) Registered pharmacists (iv) Registered nurses
Terms of use
PP-UNP-IRL-0901. July 2025
PP-UNP-IRL-0901. July 2025