This site is intended only for healthcare professionals resident in the Republic of Ireland
Menu
Close
Menu
Close
The information on this website is based on data from adult patients with ALK-positive advanced NSCLC (anaplastic lymphoma kinase positive advanced non small cell lung cancer) treated with LORVIQUA▼(lorlatinib), produced in line with the LORVIQUA (lorlatinib) Summary of Product Characteristics.
LORVIQUA (lorlatinib) Prescribing Information click here.
▼LORVIQUA is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions. Adverse event reporting information can also be found at the bottom of the page.
LORVIQUA was evaluated as a first-line treatment option in the CROWN study. CROWN was a global, randomised, open-label, multicentre, Phase 3 trial of LORVIQUA vs crizotinib in patients with previously untreated, ALK-positive, locally advanced or metastatic NSCLC (N=296).1,2
Crizotinib was selected for the comparator arm because it was the standard-of-care first-line treatment at the time of trial initiation.2
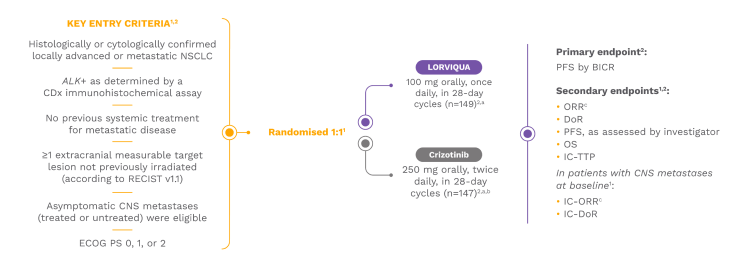
Approximately 26% of patients in both the LORVIQUA and crizotinib treatment arms had brain metastases at baseline.2
| Characteristic |
LORVIQUA (n=149) |
Crizotinib (n=147) |
|---|---|---|
| Age, years | ||
| Mean (SD)d | 59.1 (±13.1) | 55.6 (±13.5) |
| Median (IQR) | 61 (51-69) | 56 (45-66) |
| Sex, n (%) | ||
| Female | 84 (56) | 91 (62) |
| Male | 65 (44) | 56 (38) |
| Race or ethnic group, n (%)e | ||
| White | 72 (48) | 72 (49) |
| Asian | 65 (44) | 65 (44) |
| Black | 0 | 1 (1) |
| Missing | 12 (8) | 9 (6) |
| Smoking status, n (%)f | ||
| Never smoked | 81 (54) | 94 (64) |
| Previous smoker | 55 (37) | 43 (29) |
| Current smoker | 13 (9) | 9 (6) |
| ECOG PS, n (%)g | ||
| 0 | 67 (45) | 57 (39) |
| 1 | 79 (53) | 81 (55) |
| 2 | 3 (2) | 9 (6) |
| Characteristic | LORVIQUA (n=149) |
Crizotinib (n=147) |
|---|---|---|
| Current stage of disease, n (%) | ||
| IIIA | 1 (1) | 0 |
| IIIB | 12 (8) | 8 (5) |
| IV | 135 (91) | 139 (95) |
| Otherh | 1 (1) | 0 |
| Histologic type, n (%) | ||
| Adenocarcinoma | 140 (94) | 140 (95) |
| Adenosquamous carcinoma | 6 (4) | 5 (3) |
| Large cell carcinoma | 0 | 1 (1) |
| Squamous cell carcinoma | 3 (2) | 1 (1) |
| Use of previous anticancer drug therapy, n (%)i | ||
| 12 (8) | 9 (6) | |
| Previous brain radiotherapy, n (%) | ||
| 9 (6) | 10 (7) | |
| Brain metastases at baseline, n (%) | ||
| 38 (26) | 40 (27) | |
IC-TTP=intracranial time-to-progression; IQR=interquartile range; ITT=intention to treat; NSCLC=non-small cell lung cancer; ORR=objective response rate; OS=overall survival; PFS=progression-free survival; RECIST=Response Evaluation Criteria in Solid Tumours; SD=standard deviation.
Pfizer. LORVIQUA (lorlatinib) Summary of Product Characteristics.
Adverse events should be reported.
If you wish to make a medical information inquiry or report an adverse event please contact Pfizer on 1800 633 363
or email Pfizer at [email protected] or visit www.PfizerMedicalInformation.ie
Report an adverse event to your national reporting system (HPRA Pharmacovigilance)
Please sign in or register to gain access to information relating to Pfizer medicines and vaccines, medical conditions, patient materials and services.
This site is intended only for healthcare professionals resident in the Republic of Ireland. If you are a member of the public wishing to access information on a specific medicine, please visit https://www.medicines.ie
This website is brought to you by Pfizer Healthcare Ireland Unlimited Company, The Watermarque Building, Ringsend Road, Dublin 4, Dublin, Ireland, D04 K7N3. Registered in the Republic of Ireland No. 127002. Directors: D. Mangone (Managing), O. Gavan, D. Kennedy. Company Secretary: M.Byrne.
Copyright © 2024 Pfizer Limited. All rights reserved.
The information on this site is reserved exclusively for healthcare professionals resident in the Republic of Ireland and contains promotional content.
I confirm that I am a healthcare professional* resident in the Republic of Ireland.
If you select 'No', you will be redirected to Pfizer.ie, where you will be able to access information on Pfizer Healthcare Ireland Unlimited Company.
*The IPHA Code definition of a healthcare professional is a person of any of the following classes: (i) Registered medical practitioners (ii) Registered dentists (iii) Registered pharmacists (iv) Registered nurses
Terms of use
PP-UNP-IRL-0891. February 2025
PP-UNP-IRL-0891. February 2025